
Note that the larger atoms (based on the size of the spheres) tend to have the smallest IE values. You may recall that the role of d orbitals in bonding in main group compounds with coordination numbers of 5 or higher remains somewhat controversial.\): Ionization energy on the periodic table. Consequently, electron configurations with more than four electron pairs around a central, second-period element are simply not observed. The energy of the 3d orbitals far exceeds the energy of the 2s and 2p orbitals, so using them in bonding is energetically prohibitive. (a) The covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as Cl 2. As an example, LiCl, which is partially covalent in character, is much more soluble than NaCl in solvents with a relatively low dielectric constant, such as ethanol (ε = 25.3 versus 80.1 for H 2O).īecause d orbitals are never occupied for principal quantum numbers less than 3, the valence electrons of second-period elements occupy 2s and 2p orbitals only. Atomic radii are often measured in angstroms (), a non-SI unit: 1 1 × 1010 m 100 pm. As such, the bonding in such compounds has a significant covalent component, giving the compounds properties that can differ significantly from those expected for simple ionic compounds. The very small cations derived from second-period elements have a high charge-to-radius ratio and can therefore polarize the filled valence shell of an anion. describe variations that occur in the general trend of atomic size in the transition metals. use the general trends to predict the relative sizes of atoms. describe the general trend in atomic size for groups and periods. The number of electrons in the atoms of the elements. Atomic radius increases as you go down the Group 1 elements. describe the factors that determine the trend in atomic size. The approximate order of stability of the successive subshells in an atom is indicated in the chart below.
Because of the smaller atomic size, simple binary ionic compounds of second-period elements also have more covalent character than the corresponding compounds formed from their heavier congeners. (A) Trends in the Atomic Radius of Group 1 (IA, Alkali Metals) Elements. Thus BF 3 forms only the four-coordinate, tetrahedral BF 4 − ion, whereas under the same conditions AlF 3 forms the six-coordinate, octahedral AlF 6 3 − ion. Moreover, the small sizes of these elements prevent them from forming compounds in which they have more than four nearest neighbors. (b) The metallic atomic radius, rmet, is. Vocabulary anion cation ion Introduction An atom is electrically neutral, which means that the number of protons is equal to the number of electrons.

use the general trends to predict the relative sizes of ions. describe the trend in ionic size for elements. Figure 2.8.2: Definitions of the Atomic Radius. describe the factors that determine the trend in ionic size. (a) The covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as Cl 2. Atomic radii are often measured in angstroms (), a non-SI unit: 1 1 × 1010 m 100 pm. When an electron is added to such a small atom, increased electron–electron repulsions tend to destabilize the anion. Atomic radii are often measured in angstroms (Å), a non-SI unit: 1 Å 1 × 1010 m 100 pm. Element \text B B has more electron-occupied shells than element \text A A. In contrast to the chemistry of the second-period elements, the chemistry of the third-period elements is more representative of the chemistry of the respective group.ĭue to their small radii, second-period elements have electron affinities that are less negative than would be predicted from general periodic trends. Elements \text A A & \text B B belong to the same group. The semimetals lie along the diagonal line separating the metals from the nonmetals and exhibit intermediate properties. Consequently, the elements in the upper right of the periodic table are the smallest and most electronegative the elements in the bottom left are the largest and least electronegative. In contrast, atomic size decreases from left to right and from bottom to top.

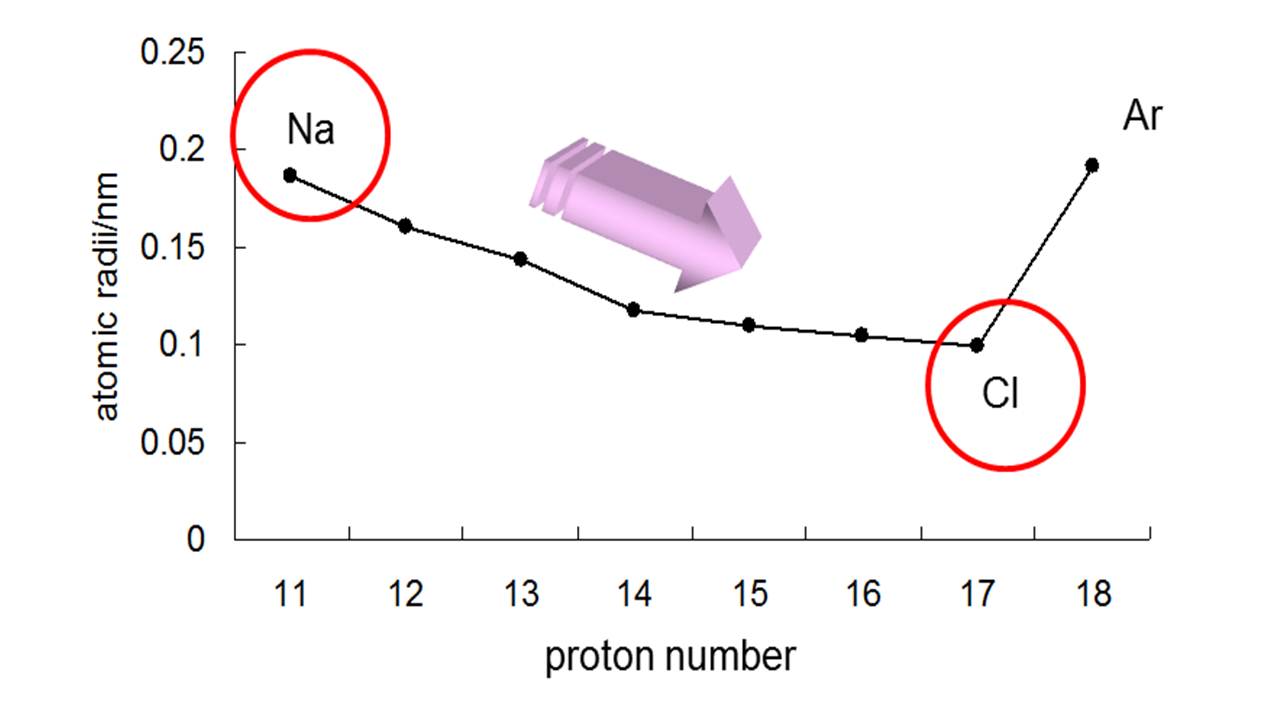
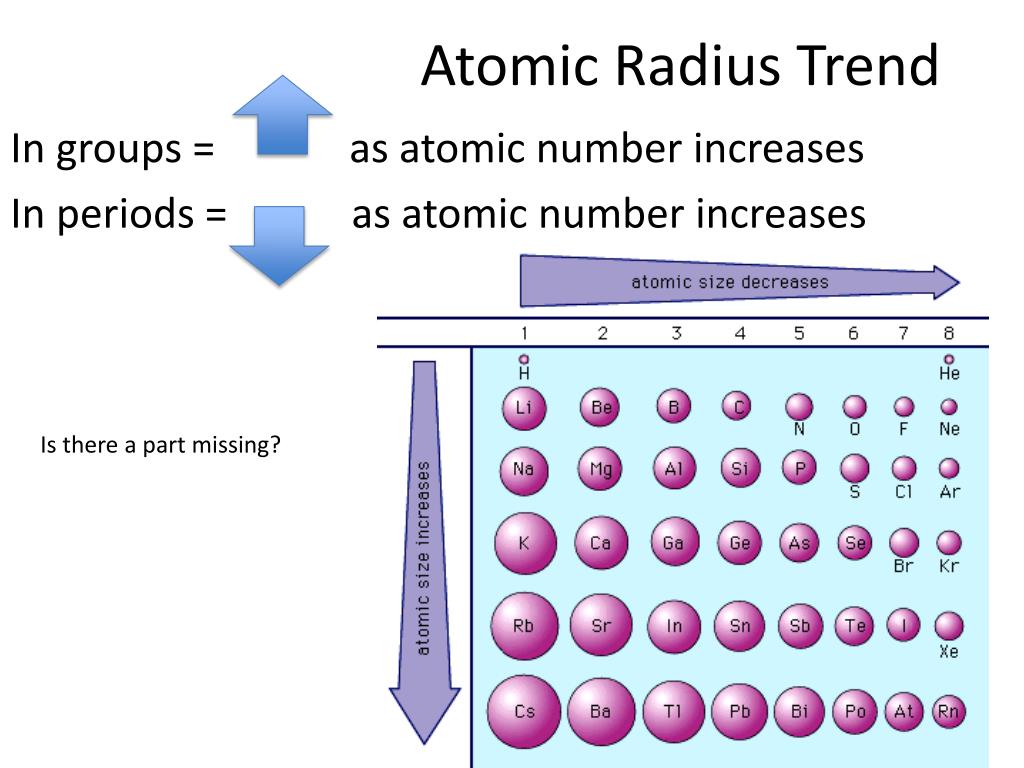
As can be seen in the figures below, the atomic radius increases from top to bottom in a group. Ionization energies, the magnitude of electron affinities, and electronegativities generally increase from left to right and from bottom to top. Atomic radii vary in a predictable way across the periodic table. \): Summary of Periodic Trends in Atomic Properties.


 0 kommentar(er)
0 kommentar(er)
